4DFix
Partially absorbable parietal reinforcement implant
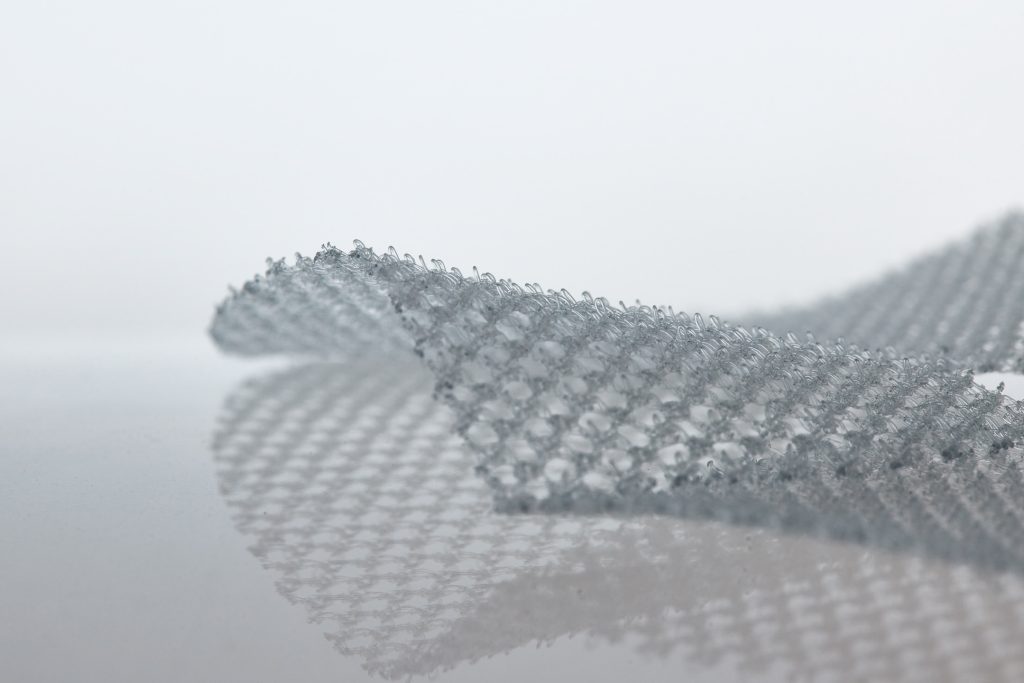
4DFix
Partially absorbable parietal reinforcement implant
Descriptions
Technical specifications
Descriptions
Partially resorbable polypropylene (35%) parietal reinforcement implant with PLLA loops (65%).
These loops provide a hydrophilic mechanism that limits the need for traditional fixation in inguinal and femoral hernia repair. 4DFix is also easily repositionable intraoperatively. The prosthesis can be cut to suit the surgeon’s needs and the patient’s anatomy.
Technical specifications
Compostition
› 65% PLLA – resorbable
› 35% polypropylene – non resorbable
› 105 g/m² weight before PLLA resorption
› 30 g/m² weight after resorption
Legal notice: 4DFix is a class III medical device manufactured by COUSIN BIOTECH S.A.S. The CE conformity has been carried out by the notified body DQS MED (CE0297). The management system of COUSIN BIOTECH S.A.S is certified for compliance with ISO 13485 (2016) standard. Please read carefully the instructions for use before using the device.
Cousin Biotech is the legal manufacturer of the medical devices proposed by Cousin Surgery.
| Ref. | Size (cm) |
| VCBFX613RU (Right) / VCBFX613LU (Left) | 6 x 13,5 |
| VCBFX0812U | 8,5 x 12,5 |
| VCBFX812FU | 8 x 12 |
| VCBFX1215U | 12 x 15 |
| VCBFX1317U | 13 x 17 |
| VCBFXLAPRU (Right) / VCBFXLAPLU (Left) | 12 x 16,5 |
| VCBFXPRSRU (Right) / VCBFXPRSLU (Left) | 10,5 x 14 |
| VCBFXPRMRU (Right) / VCBFXPRMLU (Left) | 12 x 15 |
| VCBFXPRLRU (Right) / VCBFXPRLLU (Left) | 12 x 17 |
| VCBFX1717U | 17 x 17 |

